Ester Bron Leaks - Uncovering Chemical Secrets
Have you heard whispers about “Ester Bron leaks” floating around? Perhaps you've seen the phrase and wondered what on earth it could mean. Well, as a matter of fact, it's not quite what you might think. This isn't about some secret celebrity mishap or a hidden scandal, not in the way you might assume, anyway. Instead, we're here to pull back the curtain on a different kind of “leak” – a fascinating peek into the world of chemistry, specifically about a group of compounds called esters.
You see, sometimes the most interesting discoveries aren't about people, but about the very building blocks that make up our world. What we're talking about today, arguably, is a sort of revelation, a sharing of insights about these chemical structures that are, in some respects, all around us. It's about getting to know these compounds better, really, and understanding their quiet yet significant presence.
So, forget the gossip, because today's conversation is about something far more fundamental and, honestly, quite cool. We're going to talk about what esters are, how they come to be, and why they matter in our daily lives. It's like uncovering the true story behind a rather intriguing name, giving you the real scoop on these chemical players.
Table of Contents
- What is an Ester, Really?
- How Do These 'Ester Bron Leaks' Happen?
- What Do Esters Look Like Up Close?
- Where Do We See Esters Everyday?
- Are All Esters the Same?
- Can We Make Esters Ourselves?
- What Happens When an Ester 'Leaks' or Breaks Down?
- Why Do These 'Ester Bron Leaks' Matter?
What is an Ester, Really?
When we talk about an ester, we're generally referring to a particular kind of chemical combination. Picture it this way: it’s a compound that starts its life from an acid. Now, this acid could be one of those organic types, which basically means it's carbon-based and often found in living things or things that once lived. Or, it could be an inorganic acid, which is more about minerals and non-living sources. What happens, pretty much, is that a tiny, single hydrogen piece from a specific spot on that acid – a part called a hydroxyl group, which is an oxygen and a hydrogen joined together – gets swapped out. Something else comes in and takes its place, creating this new, distinct compound we call an ester. It’s like a little chemical exchange, a sort of trade-off, if you will, that results in something new.
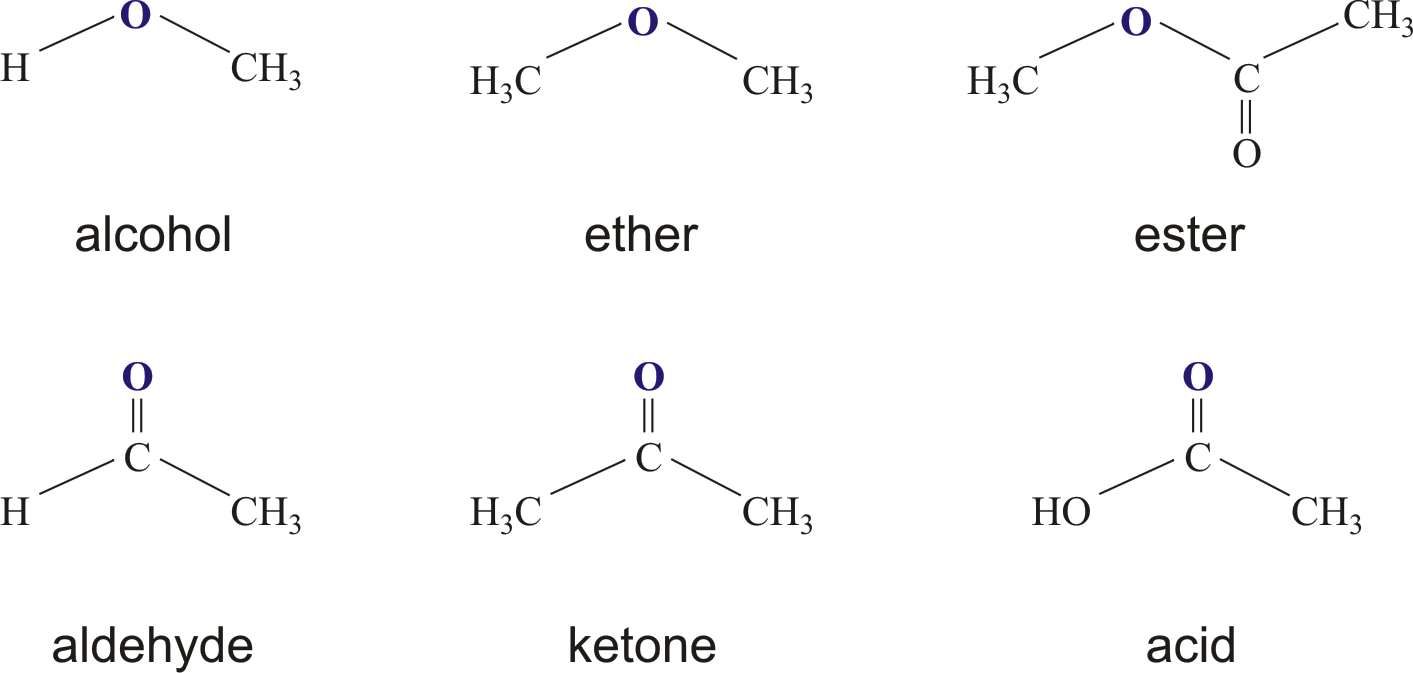
These esters are, in essence, a big family of compounds that have carbon as their backbone. They’re rather interesting because they have a particular way of interacting with water. When an ester and water meet, they don't just mix; they actually react. This reaction causes them to break apart, and when they do, they turn into two different things: an alcohol and then, you know, either an organic acid or an inorganic acid, depending on what they started as. It’s a bit like taking apart a toy to see its individual pieces, revealing the components it was built from.
Now, among all the different kinds of esters out there, the ones that come from what we call carboxylic acids are, by far, the most common ones you'll encounter. These carboxylic acids are a very important group in chemistry, known for having a particular arrangement of atoms, and they serve as the usual starting point for making many esters. So, if you hear someone talking about an ester, chances are, they're referring to one that got its start from a carboxylic acid, actually.
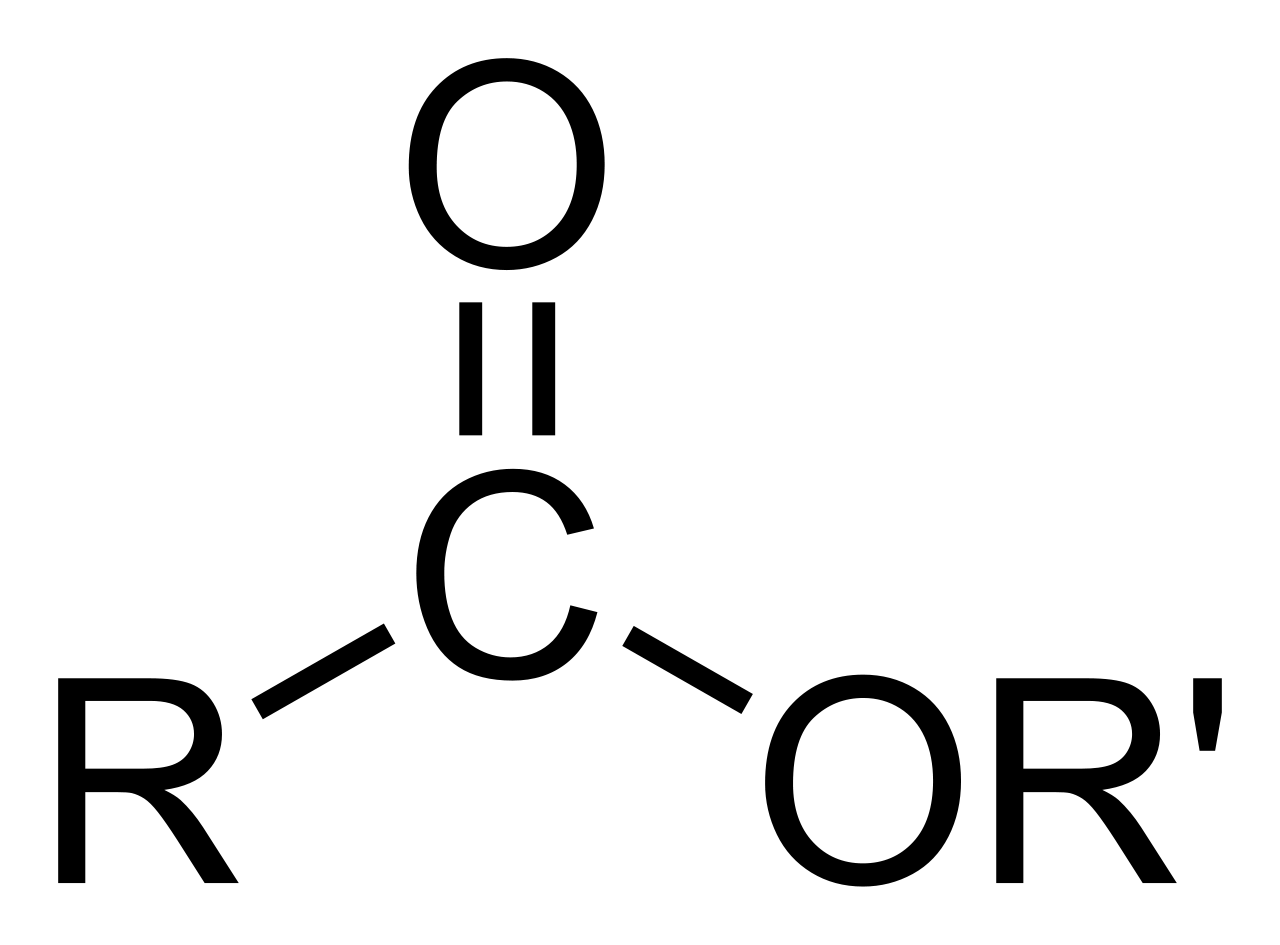
A key idea to keep in mind, a pretty fundamental characteristic, is how an ester is put together. At its heart, an ester has a carbon atom that's double-bonded to an oxygen atom – this part is called a carbonyl group. And then, from that very same carbon atom, there's another oxygen atom attached. This second oxygen atom is then connected to another carbon group. This specific arrangement of atoms is what makes an ester, well, an ester. It’s like a unique signature, a specific pattern of connections that defines it.
So, in short, an ester is an organic compound where a hydrogen atom that would normally be part of a carboxyl group – a specific acid-like part of a molecule – has been swapped out for a hydrocarbon group, which is basically a chain or ring of carbon and hydrogen atoms. This replacement is what gives esters their distinct qualities and allows them to do all sorts of different things. They are, in a way, cousins to carboxylic acids, sharing some family traits but having their own unique twists.
How Do These 'Ester Bron Leaks' Happen?
When we talk about "leaks" in the context of esters, it’s not about secrets spilling, but rather about how these compounds come into being or how they break down. You could say it’s about the ways their internal structure is revealed or altered. One way an ester is made, pretty much, is through what chemists call a condensation reaction. Picture this: you take an acid, usually an organic one, and you combine it with an alcohol, or perhaps a phenol compound, which is a similar type of chemical. When these two get together, a small molecule of water is actually lost, it "leaks" away, and the remaining pieces join up to form the ester. It’s a bit like two puzzle pieces fitting together perfectly, with a tiny bit of material being released in the process. This is one of the most common ways these compounds are put together, though, to be honest, there are other methods too.
The names we give to esters, you know, are also a bit of a clue to their structure. They often include prefixes that tell you about the length of the carbon chains within the molecule. It's like giving them a first name and a last name, where the first part tells you about one piece of the molecule and the second part tells you about another. This naming system helps chemists, and us, get a quick idea of what the ester might look like, more or less, just from its name.
And speaking of how they're put together, the very connection that defines an ester – that specific arrangement of carbon and oxygen atoms – is something we find in a lot of natural things. For example, it’s present in animal fats, which are a really important part of our diet and biology. Beyond that, it’s found in many molecules that are, quite simply, vital for life. So, these "ester bron leaks" of information about their structure show us just how fundamental they are to the world around us, and even inside us, in a way.
What Do Esters Look Like Up Close?
If you were to zoom in really close on an ester, down to its atomic makeup, you'd see a very specific arrangement of atoms. As we mentioned, there's a carbon atom that forms a strong connection, a double bond, with one oxygen atom. This pairing is a central part of the ester's identity. Then, from that same carbon, another oxygen atom reaches out and forms a bond. This second oxygen atom is, in turn, linked to another carbon atom, which is part of a larger chain or group of atoms. This chain or group can vary quite a bit, making each ester unique, yet still part of the same family. It's like having a core structure that's always the same, but with different branches extending from it, giving each one its own character. This visual, this picture of how the atoms are arranged, helps us understand why esters behave the way they do, and what sorts of properties they might have, generally.
The names given to esters are, actually, quite descriptive of their physical makeup. These names usually have parts, or prefixes, that tell us about the size of the carbon chains within the molecules. For instance, one part of the name might tell you about the alcohol component that went into making the ester, while the other part describes the acid component. This naming convention is a pretty clear way to communicate the molecular structure without having to draw it out every time. It's like a code that, once you know it, reveals a lot about the compound's building blocks, and stuff.
Understanding this structure is, in some respects, key to understanding why esters are used for so many different things. Their specific arrangement of atoms gives them properties like being able to dissolve certain substances, or having distinct smells. It’s this precise molecular architecture that allows them to perform their various roles, whether it's as a solvent, a flavor, or a building block in something bigger. So, knowing what they look like up close, really, helps us appreciate their versatility.
Where Do We See Esters Everyday?
You might not realize it, but esters are, quite literally, all around us, and in many things we use or experience daily. The chemical industry, for example, relies on esters for a whole bunch of different purposes. They're like versatile tools in a chemist's toolkit, capable of doing many jobs. For instance, ethyl acetate, which is a type of ester, is a very common solvent. You'll find it in things like nail polish remover, where it helps dissolve the polish, or in glues, where it helps keep things stuck together. It’s pretty much everywhere, once you start looking, because of its ability to dissolve other substances so well.
Beyond industrial uses, esters are also responsible for many of the pleasant smells and tastes we enjoy. Think about the sweet smell of ripe fruit – that's often due to esters. The distinct aroma of bananas, apples, or pineapples? Those are, in fact, esters at work. They give fruits their characteristic scents and flavors, making them appealing to us. So, the next time you enjoy a fruit, you’re experiencing the effects of these fascinating compounds, in a way.
Moreover, as we briefly touched on, the ester connection is not just in man-made products or fruit. It’s also a big part of natural, living systems. Animal fats, for example, are essentially complex esters. And many other molecules that are absolutely vital for life – things that help our bodies function or that are part of biological processes – also contain these ester linkages. So, from the food we eat to the way our bodies work, esters play a pretty significant, if often unseen, role, sometimes.
They’re also used in making plastics, textiles, and even some medicines. Their versatility comes from their ability to be modified, to have different carbon chains attached, which changes their properties. This means chemists can, more or less, design esters for specific jobs, whether it's to make a plastic more flexible or to create a particular scent for a perfume. It's really quite remarkable how adaptable these compounds are, allowing them to fit into so many different aspects of our lives.
Are All Esters the Same?
No, not at all, actually. While all esters share that basic core structure – the carbon double-bonded to an oxygen, and then another oxygen connected to another carbon group – the parts attached to that core can vary quite a bit. This variation is what gives each ester its own unique identity and properties. Think of it like a family, where everyone shares a last name, but each person has their own first name and personality. The general formula for an ester is often written as RCO2R′, where 'R' and 'R
- Chocolate Models Jean
- Https Onlyfans Com Omgjasmin
- Leo Gold Nudes
- Syren De Mer Selfie
- How Old Is N8noface
Ester Definition, Examples And Facts | Chemistry Dictionary
Esters @ Chemistry Dictionary & Glossary
Ester - Chemistry LibreTexts