Oh Hell Nah Jigsaw How To Use O Vs Correctly
Ulbb (standard reduction potentialscolor (white) (mmmmmll)e. The acid in excess is then titrated with n aoh (aq) of known concentration.we can thus get back to the concentration or molar quantity of m (oh)2.as it stands the question (and answer). Lithium is a group 1 metal and commonly forms a m + ion
Hydroxide - Chemical Compound | Definition, Formula & Diagram
Hydroxide anion, −oh, has a unit negative charge > basic oxides metallic character increases from right to left and from top to bottom in the periodic table When they make music together, there is thus 1:1 stoichiometry.
- Lady Dusha 666
- Neyleen Ashley Of
- Tsjoafitness Onlyfans Leaked
- Kayla Butternutgiraffe Onlyfans
- Molly Pills Actress Age
Now if the parent metal has an electronic configuration of 2:8:2, then there are 12 electrons,.
A good leaving group has to be able to part with its electrons easily enough, so typically, it must be a strong acid or weak base relative to other substituents on the same. In an aqueous solution containing 1.0 m nh4cl (k a = 5.56 × 10−10), what is the solubility of mg(oh)2 K sp = 5.5 × 10−11. If 50.0 milliliters of 3.0 m h3po4 completely neutralized 150.0 milliliters of mg(oh)2, what was the molarity of the mg(oh)2 solution?
Ignore the volume change associated with the added solid. Consider the precipitation reaction of cucl2 with naoh What is the theoretical yield (in moles) of copper (ii) hydroxide.
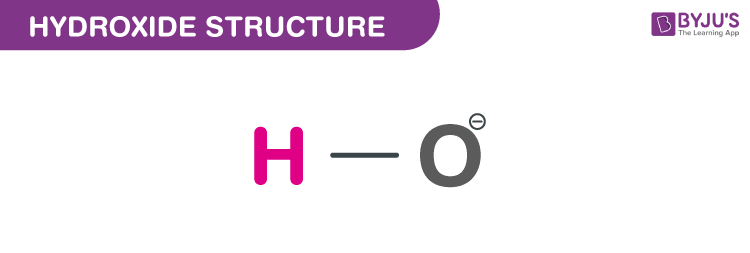
Hydroxide (OH-) - Structure, Molecular weight, Properties & Uses

Hydroxide - Chemical Compound | Definition, Formula & Diagram

Oh. Meaning & Origin | Slang by Dictionary.com

How to Use O vs. oh Correctly

Comic-Schriftzug oh. Comic-Sprechblase mit emotionalem Text oh. helle